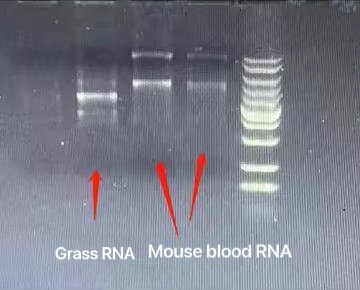
Step 1. Place the filter membrane

Step 2. Pour in water sample and start filtration

Step 3. Filtration completed

Step 4. Remove the filter membrane

Step 5. Roll the filter membrane inward and insert into a 5ml grinding tube


Step 6. Add grinding beads to the center of the grinding tube
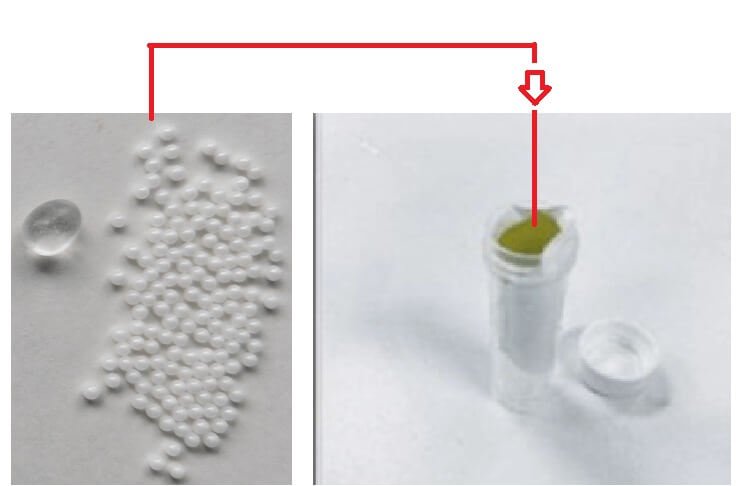
Step 7. Proceed with subsequent grinding and extraction operations according to the DNK46 FastBeat Water DNA Kit (Bead Beating) protocol (as follows)
- Add 980 μl Sodium Phosphate Buffer to sample in Bead Tube. Gentle vortex to mix. Add 120 μl MT Buffer.
Note: Check MT Buffer. If MT Buffer is precipitated, heat solution to 60°C until dissolved before use. - Homogenize in the FastPrep® Instrument for 40 seconds at a speed setting of 6.0.
- Centrifuge at 12,000 x g for 5minutes to pellet debris.
- Transfer supernatant to a clean 2.0 ml centrifuge tube. Add 250μl PPS Solution and mix by shaking the tube by hand 10 times. Incubate at 4°C for 5 minutes.
- Centrifuge tubes at 10,000 x g for 3 minute at room temperature. Avoiding pellet, transfer up to, but no more than, 900 μl of supernatant to a clean 2 ml centrifuge tube.
- Add 300 μl of IRS Solution(1/3 volume) and vortex briefly. Incubate at 4°C for 5 minutes.
- Centrifuge tubes at 10,000 x g for 1 minute at room temperature. Avoiding pellet, transfer the supernatant into a clean 5 ml centrifuge tube.
- Add 1.5 volumes of PQ Solution to the cleared supernatant and mix by pipetting.
Example: To 1100 μl lysate add 1650 μl PQ Solution. Reduce the amount of PQ Solution accordingly if less supernatant is recovered. A precipitate may form after the addition of ethanol but this will not affect the procedure.
Note: Ensure ethanol has been added to PQ Solution.
Note: It is important to pipet PQ Solution directly onto the cleared supernatant and to mix immediately. - Load approximately 700 μl mixture onto Spin Filter(sitting in collection tube) and centrifuge at 10,000 x g for 1 minute at room temperature. Discard flow through. Load another 700 μl and repeat until all remaining mixture is loaded on Spin Filter.
Note: A total of 4-5 loads for each sample processed may be required. - Add 600 μl of Buffer WB to Spin Filter and centrifuge at 10,000 x g for 30 seconds at room temperature. Discard flow through. Repeat Step 10 with another 600 µl Buffer WB.
Note: Ensure ethanol is added to Buffer WB. - Centrifuge Spin Filter at 13,000 x g for 2 minute at room temperature to dry the Spin Filter..
- Carefully place Spin Filter in clean 1.5 ml centrifuge tube. Avoid splashing any Buffer WB onto Spin Filter. Add 100 µl of Elution Buffer (Optional: pre-warm the water to 70–90℃ will increase the DNA yield) to the center of the column membrane. Incubate at room temperature for 3-5 min, and centrifuge at 13,000 x g for 1 min to elute the DNA.
Note: Use smaller volume(minimum 30µl) of Elution Buffer will obtain higher concentration.
Optional: Put eluate back to the Spin Column to repeat elution once. This increases concentration of DNA about 10-15%